Patients across the U.S. have filed lawsuits against Cartiva Inc., Wright Medical Group, and Stryker, alleging that the companies concealed known defects in the Cartiva toe implant and continued to market it despite evidence of significant risks. The complaints claim the manufacturers misled doctors and patients, which led to painful complications and, in many cases, the need for Cartiva revision surgery.
As court filings mount, the litigation could soon develop into a broader Cartiva lawsuit and potentially pave the way for consolidated proceedings and future settlements.
What Is the Cartiva Toe Implant?
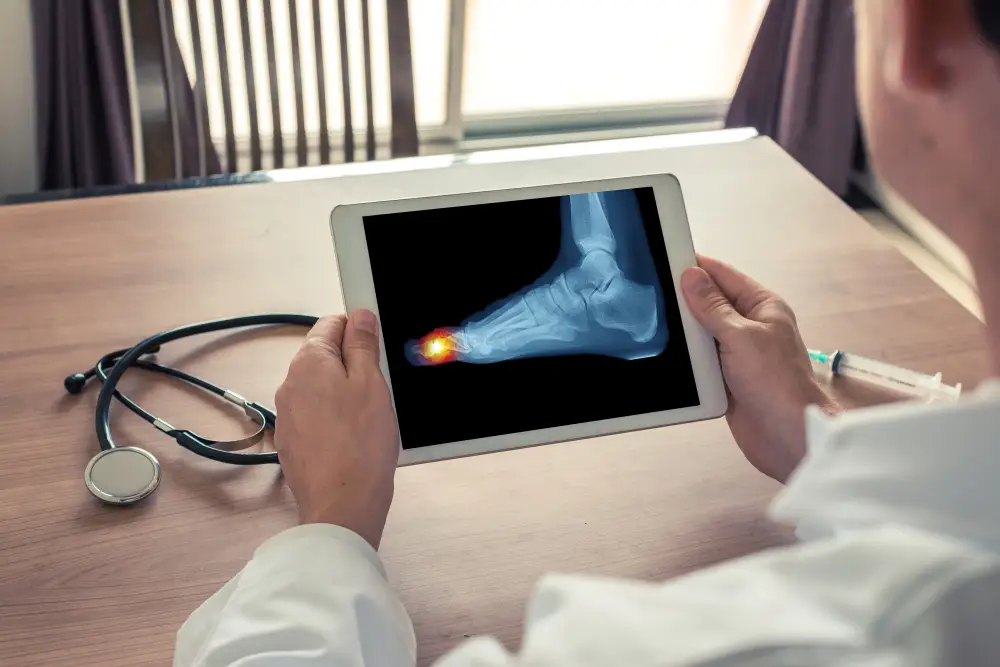
The Cartiva implant is a synthetic cartilage device primarily designed to relieve toe arthritis pain in the big toe joint, known medically as the first metatarsophalangeal (MTP) joint, while preserving natural motion. Marketed as a less invasive alternative to fusion surgery, it was approved by the U.S. Food and Drug Administration (FDA) in 2016. The implant is made of a hydrogel polymer composed of polyvinyl alcohol and saline, intended to mimic the cushioning properties of natural cartilage.
However, reports from patients and physicians indicate that the implant has not lived up to expectations. A growing number of individuals allege that the device fails prematurely, sometimes within months after surgery, resulting in pain, swelling, stiffness, and limited mobility.
Widespread Reports of Cartiva Failure
Patients describe a range of Cartiva implant complications, including the device shrinking, loosening, or migrating deeper into the bone. These issues can lead to loss of motion, inflammation, nerve damage, and, in severe cases, infection or bone cyst formation.
According to a physician involved in the implant’s early development, “There was something wrong,” prompting him to stop recommending the product altogether. Even major insurer Cigna has labeled the procedure as “experimental,” citing insufficient long-term data.
While initial FDA filings suggested a Cartiva failure rate of around 13%, some real-world studies tell a different story. A study published in Foot & Ankle Orthopedics reported a Cartiva implant failure rate as high as 64%.
These concerns have fueled hundreds of lawsuits alleging medical device failure, defective design, and inadequate warnings to both patients and physicians.
The 2024 Cartiva Recall and Manufacturer Response
In October 2024, Stryker, which owns Cartiva Inc., issued an urgent medical device recall for the Cartiva Synthetic Cartilage Implant (SCI). The company acknowledged new postmarket data showing higher-than-expected rates of implant subsidence, displacement, pain, and fragmentation.
The recall notice instructed surgeons and hospitals to quarantine and return affected products distributed between July 2016 and October 2024. It also urged doctors to monitor existing patients for symptoms such as swelling, stiffness, or difficulty walking, which are common signs of Cartiva implant complications.
Despite the Cartiva recall, lawsuits assert that manufacturers were aware of serious issues long before the public was notified and failed to take timely corrective action.
Key Cases and Recent Legal Developments
May v. Cartiva, Inc. et al.
Filed in the U.S. District Court for the Southern District of West Virginia (Case No. 2:24-cv-00687), May v. Cartiva is handled by Judge Irene C. Berger, who has set an extensive pretrial schedule, with discovery ongoing through early 2026 and a trial set for August 3, 2026.
Peachey v. Cartiva, Inc.
Another high-profile case involving Cartiva, Peachey v. Cartiva (Case No. 2:25-cv-01217), is pending in the Western District of Pennsylvania before Judge Christy Criswell Wiegand. Court records show ongoing discovery and mediation discussions, with an initial case management conference scheduled for November 5, 2025.
DiDonato v. Cartiva, Inc.
Filed earlier in 2025 (Case No. 2:25-cv-00187), DiDonato v. Cartiva also involves claims of premature Cartiva failure and has been referred to mediation under Judge Wiegand. Judge Lisa Lenihan was appointed mediator in October 2025 as the parties discussed potential resolution options.
Hughes v. Cartiva, Inc.
The first-ever Cartiva SCI trial, Hughes v. Cartiva (Case No. 2:24-cv-00319), initially set for October 28, 2025, was postponed to May 26, 2026. Plaintiff Bryan Hughes alleged device failure and claims manufacturers concealed prior adverse event reports.
These cases, along with others pending across the country, are shaping what could become a large-scale Cartiva multidistrict litigation (MDL) if federal courts agree to consolidate similar claims.
The Question of Settlement and Compensation
As of late 2025, no official Cartiva settlement has been announced. However, court observers note that some recently filed cases have been quietly dismissed, fueling speculation that confidential agreements or tolling arrangements may be underway.
If future settlements occur, they may provide Cartiva compensation for medical expenses, lost wages, pain and suffering, and other losses related to the defective implant.
For now, potential plaintiffs are encouraged to document symptoms, surgical records, and any subsequent Cartiva revision surgery to preserve their right to claim compensation if a Cartiva settlement is later approved.
Who May Qualify to File a Cartiva Lawsuit?
Individuals may be eligible to join a potential Cartiva class action lawsuit if they:
- Received a Cartiva toe implant between 2016 and 2024
- Experienced early Cartiva implant failure or complications
- Required Cartiva revision surgery or fusion surgery after the implant failed
- Suffered nerve damage, ongoing pain, or limited joint movement
What Happens Next?
With litigation expanding and evidence mounting, many expect that a consolidated MDL could be established to streamline discovery and trial processes. If granted, this would group similar cases before one federal judge, potentially helping determine whether the manufacturer bears responsibility for the widespread failures of its foot and ankle implant.
Although the settlements in the Cartiva lawsuits have not yet materialized, discussions appear to be underway. Legal analysts suggest that resolution efforts could accelerate once expert discovery is completed in 2026.
Frequently Asked Questions (FAQ)
The Cartiva implant is a synthetic cartilage device used to treat toe arthritis, designed to relieve pain in the big toe joint while maintaining motion.
Patients allege that the implant was defectively designed, leading to high rates of Cartiva failure and the need for Cartiva revision surgery or fusion surgery.
Yes. In October 2024, Stryker issued a Cartiva recall due to reports of higher-than-expected failure rates and serious post-surgical complications.
No formal Cartiva settlement has been announced, though ongoing case dismissals suggest that confidential agreements may be in progress.



Add Comment